World First Gene Therapy Restores Hearing in Patients with Genetic Deafness
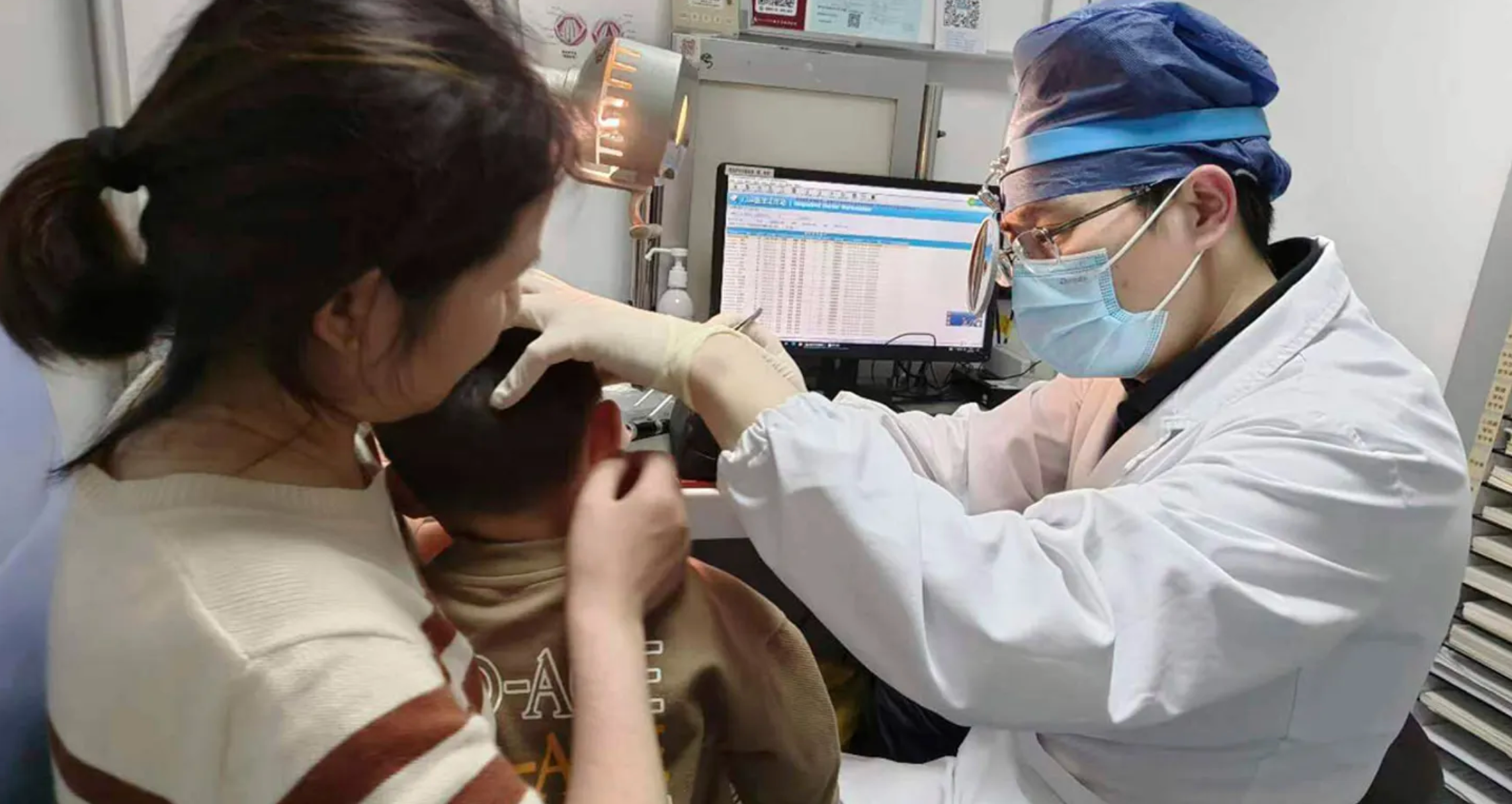
In a breakthrough for treating genetic deafness, a research team led by the Department of Otorhinolaryngology at Fudan University Affiliated Eye and ENT Hospital has successfully used experimental gene therapy to restore hearing and speech in patients with genetic hearing loss. The clinical study results were published in The Lancet on January 25th.
Congenital deafness affects 26 million people worldwide. In China alone, around 30,000 children are born deaf every year, and approximately 60% of cases are linked to genetic factors. This severely hinders children’s speech, cognitive, and intellectual development. Currently, there are no approved medications to treat genetic deafness. Mutations in the OTOF gene are a common cause of congenital deafness, leading to severe to profound or total hearing loss and speech impairment in children.
With innovations in biomedical technologies, gene therapy is considered one of the most promising strategies to cure genetic deafness. By delivering vectors containing normally functioning genes directly into the inner ear, gene therapy can restore the expression of normal proteins and rescue hearing function.
To address this global challenge, Professor Shu Yilai has dedicated over ten years to developing gene therapies for deafness. After years of exploration and partnerships, his team has developed an experimental gene therapy, RRG-003, specifically targeting mutations in the OTOF gene. They also innovatively developed precise, minimally invasive methods to deliver the therapy into the ear.
Pathogenic mutations in OTOF, which encodes otoferlin, cause DFNB9, a type of auditory neuropathy where patients exhibit severe to profound hearing loss and speech disability. OTOF mutations cause up to 41% of infant auditory neuropathy cases in China. OTOF is critical in neurotransmitter release from hair cell synapses in the cochlea, enabling the brain to receive sound signals. Lack of otoferlin expression in hair cells prevents the propagation of sound stimuli to the auditory nerve pathway, resulting in deafness.
Adeno-associated viruses (AAV) are the most widely used gene therapy delivery vectors. However, the OTOF gene exceeds the cargo capacity of a single AAV. To overcome this challenge of delivering large genes into the inner ear, the team utilized a dual AAV vector system—two AAV vectors carrying portions of the OTOF gene. This restored otoferlin expression and significantly improved hearing in an OTOF-deaf animal model. As Professor Shu explains, “It’s like moving a large piece of furniture—you need two cars instead of one. After injection into the body, the two vectors combine to form the complete gene.” The team also conducted safety assessments on mouse and primate models.
Based on these foundations, Fudan University Affiliated Eye and ENT Hospital ethically approved the clinical trial in June 2022. Patient recruitment began in October 2022, followed by the world’s first gene therapy administered to a child with genetic deafness. Additional pediatric patients have since been treated. The longest follow-up is over one year, and the children can now engage in daily conversations.

In the clinical trial, six children with OTOF mutations received gene therapy injected into the inner ear via a minimally invasive approach. It demonstrated good safety and tolerability over the follow-up period. Five patients showed significant recovery of hearing and speech functions after treatment. This represents the first effective gene therapy clinical trial for deafness globally, with the most systematic approach and the largest number of cases in the field. It is also the world’s first clinical application of a dual AAV vector system, ushering in a new era of gene therapy for deafness.
This achievement is not only a triumph in medical research but also a successful example of academia-industry collaboration. The partnership between EENT Hospital and Shanghai Dingxin Gene Technology facilitated the development, production, and safety evaluation of this pioneering gene therapy, offering new hope to patients with hereditary deafness.
The clinical trial was ethically approved in June 2022 by Fudan University Affiliated Eye and ENT Hospital. Patient recruitment began in October 2022, followed by the world’s first administration of gene therapy for genetic deafness. Additional pediatric patients have since been enrolled. With over one year of follow-up completed, the children can now hold daily conversations. This represents the first effective gene therapy clinical trial for deafness globally, with the most systematic approach, the largest number of cases, and the longest follow-up time in the field.
https://www.digitimes.com/news/a20240315VL204/huawei-open-ran-china-east-asia-mobile+telecom-mobile-components-mobile-devices-telecom-service-infrastructure-wireless-networking.html




