Innovative Brain Implant Restores Mobility for Patients with Complete Paralysis
Just as Elon Musk’s Neuralink announced their success in putting an implant into a human brain, making headlines globally, China also showcases its latest progress in clinical application of brain-computer interfaces (BCIs), highlighting another emerging area of fierce competition between these 2 powers.
Brain-computer interfaces (BCIs) enable direct communication between the brain and computers by recording and interpreting brain signals. BCIs can help patients with brain disorders such as amyotrophic lateral sclerosis, spinal cord injuries, and epilepsy to recover. Moreover, they hold the potential to achieve brain-computer integration intelligence, directly expanding the information processing capabilities of the human brain.
On October 24, 2023, Professor Hong Bo from the School of Medicine at Tsinghua University led a team to design and develop the wireless minimally invasive implantable BCI called NEO (Neural Electronic Opportunity). The first clinical implantation trial was successfully conducted at Xuanwu Hospital in Beijing. On January 29, the joint team held a summary meeting for the clinical trial phase, announcing a breakthrough in the rehabilitation of the first patient with a BCI interface.
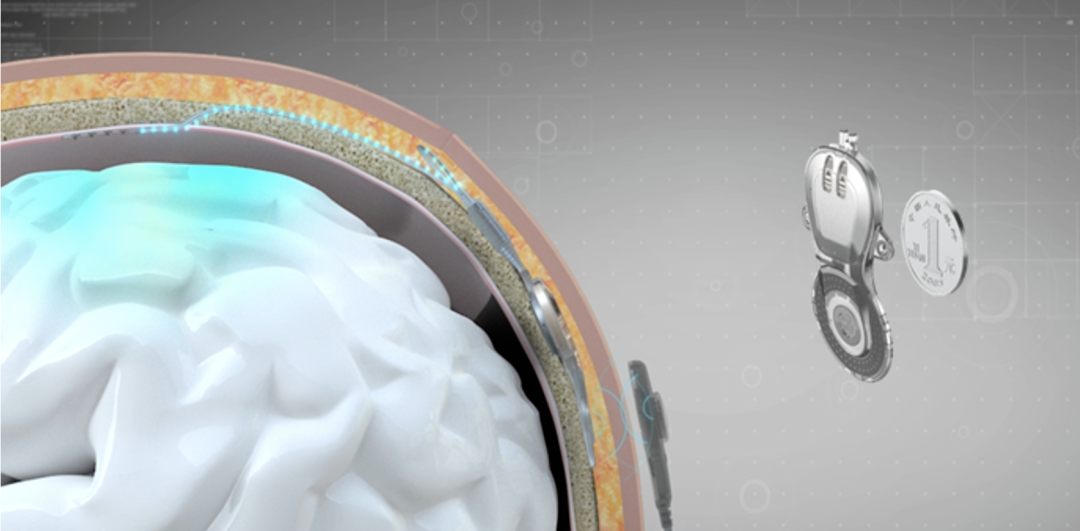
During the procedure, a brain-machine interface processor, the size of two coins, was implanted into the skull of a high-level quadriplegic patient, successfully capturing neural signals from the sensory and motor brain areas. The patient was a victim of a car accident, suffering from complete spinal cord injury at the cervical level (ASIA grade A, the highest level of paralysis), and had been paralyzed for 14 years.
This system utilizes a wireless minimally invasive design, with an implant placed inside the skull and electrodes positioned outside the dura mater (which acts as a protective layer between the skull and the brain cortex), ensuring no damage to brain cells. The patient was discharged from the hospital 10 days after the surgery.
During home use, the external device powers the implant through the scalp and receives neural signals from the brain, transmitting them to a computer or mobile phone, enabling brain-machine interface communication. The system employs near-field wireless power and communication technology, eliminating the need for batteries in the implanted internal device, allowing lifelong use for the patient.
 The team performed the first implantation.
The team performed the first implantation.
After three months of home-based rehabilitation training with the brain-machine interface, the patient was able to control a pneumatic glove through brain activity, achieving independent functions such as drinking water, with a grip accuracy rate exceeding 90%. The patient’s ASIA clinical score for spinal cord injury and sensory evoked potential responses showed significant improvement.
 First Patient Successfully Achieves Brain-Controlled Gr […]
First Patient Successfully Achieves Brain-Controlled Gr […]
Unlike the approach championed by Neuralink, this wireless minimally invasive brain-machine interface NEO places electrodes outside the brain’s dura mater, which avoids damaging neural tissue. Developed through long-term animal experiments, NEO utilizes near-field wireless power and signal transmission, eliminating the need for implanting batteries. The NEO system’s software and hardware for clinical application were developed in collaboration between the research team at Tsinghua University School of Medicine and the Suzhou-based Neuracle Technology.
It is reported that the second case of spinal cord injury patient implantation was successfully performed at Temple of Heaven Hospital in Beijing on December 19, 2023, with normal signal reception. The patient is currently undergoing home rehabilitation training. The clinical trials of this wireless minimally invasive brain-machine interface were approved by the ethics committees of Xuanwu Hospital and Temple of Heaven Hospital in April and May 2023, respectively, and have been registered for international and domestic implantable medical device clinical trials.
The development of BCI technology has great significance for both medical treatment and scientific research. It opens up new possibilities for patients with brain disorders and offers potential for enhancing human cognitive abilities. The successful clinical trial of NEO marks a significant milestone in the field of BCI research and brings hope for future advancements in this area.
https://www.digitimes.com/news/a20240315VL204/huawei-open-ran-china-east-asia-mobile+telecom-mobile-components-mobile-devices-telecom-service-infrastructure-wireless-networking.html

